Magnesium Chloride Ionic Equation . To find the formula of an ionic compound, first. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. The balanced equation will be calculated along. Web now consider the ionic compound formed by magnesium and chlorine. Web electrolysis of magnesium chloride. A magnesium ion has a 2+ charge, while a chlorine ion has a. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web enter an equation of an ionic chemical equation and press the balance button. Mgcl 2 can be extracted from.
from www.elevise.co.uk
The balanced equation will be calculated along. Web now consider the ionic compound formed by magnesium and chlorine. To find the formula of an ionic compound, first. Web electrolysis of magnesium chloride. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web enter an equation of an ionic chemical equation and press the balance button. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. A magnesium ion has a 2+ charge, while a chlorine ion has a. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Mgcl 2 can be extracted from.
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise
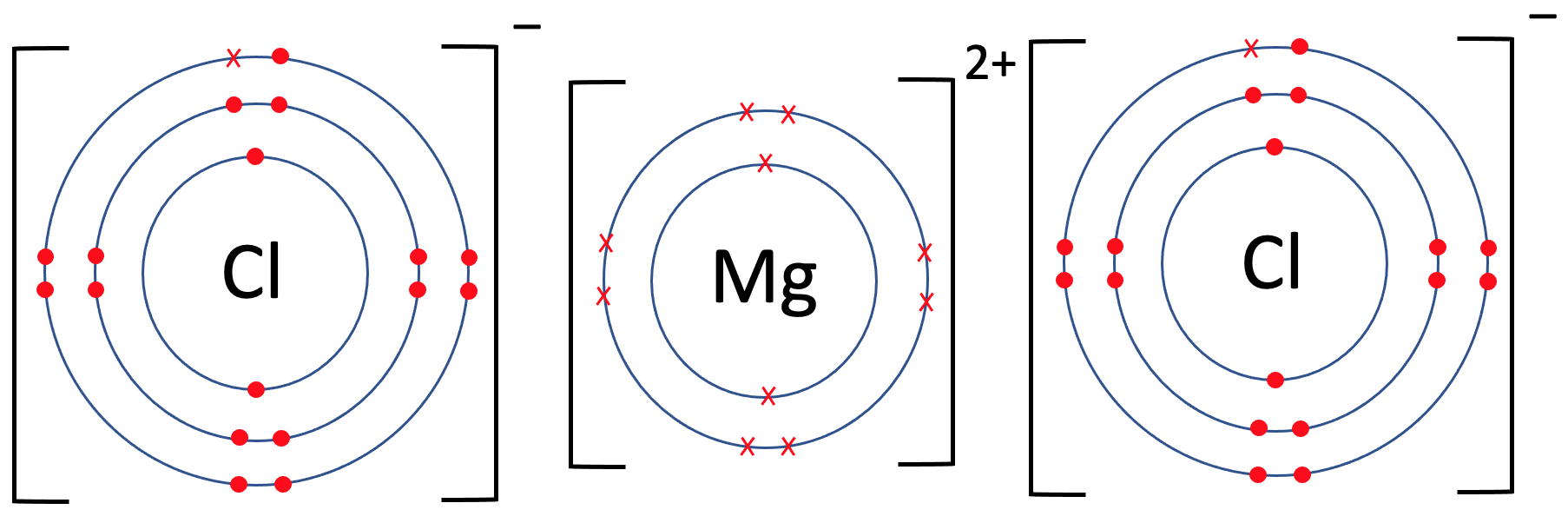
Magnesium Chloride Ionic Equation Web now consider the ionic compound formed by magnesium and chlorine. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Web electrolysis of magnesium chloride. The balanced equation will be calculated along. Web now consider the ionic compound formed by magnesium and chlorine. To find the formula of an ionic compound, first. Mgcl 2 can be extracted from. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. A magnesium ion has a 2+ charge, while a chlorine ion has a. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web enter an equation of an ionic chemical equation and press the balance button.
From diagramedia.blogspot.com
Electron Dot Diagram For Magnesium Chloride Diagram Media Magnesium Chloride Ionic Equation To find the formula of an ionic compound, first. A magnesium ion has a 2+ charge, while a chlorine ion has a. Mgcl 2 can be extracted from. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Magnesium chloride must be heated until it is molten before it will. Magnesium Chloride Ionic Equation.
From www.scibond.com
How to represent a chemical reaction? SciBond Magnesium Chloride Ionic Equation Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web now consider the ionic compound formed by magnesium and chlorine. To find the formula of an ionic compound, first. The balanced equation will be calculated. Magnesium Chloride Ionic Equation.
From jbdizkkrki.blogspot.com
Magnesium Chloride Formula Unit The Structure Of Matter Section 1 Magnesium Chloride Ionic Equation Web now consider the ionic compound formed by magnesium and chlorine. Magnesium chloride must be heated until it is molten before it will conduct electricity. Mgcl 2 can be extracted from. To find the formula of an ionic compound, first. The balanced equation will be calculated along. A magnesium ion has a 2+ charge, while a chlorine ion has a.. Magnesium Chloride Ionic Equation.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Chloride Ionic Equation Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. To find the formula of an ionic compound, first. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web now consider the ionic compound formed by magnesium and chlorine. Mgcl 2 can be extracted from.. Magnesium Chloride Ionic Equation.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Magnesium Chloride Ionic Equation Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Magnesium chloride must be heated until it is molten before it will conduct electricity. Mgcl 2 can be extracted from. Web electrolysis of magnesium chloride. To find the formula of an ionic compound, first. Web now consider the ionic compound. Magnesium Chloride Ionic Equation.
From www.coursehero.com
[Solved] write the molecular, ionic, and net ionic equation Magnesium Chloride Ionic Equation Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. Web electrolysis of magnesium chloride. To find the formula of an ionic compound, first. Web enter an equation of an ionic chemical equation and press the balance button. Mgcl 2 can be extracted from. Web a complete ionic equation is a chemical equation. Magnesium Chloride Ionic Equation.
From www.slideserve.com
PPT Honors chemistry ch . 14 Ions in Aqueous Solutions and Magnesium Chloride Ionic Equation The balanced equation will be calculated along. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Web enter an equation of an ionic chemical equation and press the balance button. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web electrolysis of magnesium chloride.. Magnesium Chloride Ionic Equation.
From byjus.com
Write the chemical formulae of the following a Magnesium chloride Magnesium Chloride Ionic Equation Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. The balanced equation will be calculated along. Mgcl 2 can be extracted from. To find the formula of an ionic compound, first. Web. Magnesium Chloride Ionic Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium Chloride Ionic Equation Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. The balanced equation will be calculated along. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web electrolysis of magnesium chloride. Web enter an equation of an ionic chemical equation and press the balance button. Web a complete. Magnesium Chloride Ionic Equation.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride Ionic Equation Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. A magnesium ion has a 2+ charge, while a chlorine ion has a. Web electrolysis of magnesium chloride. To find the formula of an ionic compound, first. Mgcl 2 can be extracted from. The balanced equation will be calculated along. Web a complete. Magnesium Chloride Ionic Equation.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Magnesium Chloride Ionic Equation Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Web now consider the ionic compound formed by magnesium and chlorine. Web enter an equation of an ionic chemical equation and press the balance button. To find the formula of an ionic compound, first. Mgcl 2 can be extracted from.. Magnesium Chloride Ionic Equation.
From www.showme.com
Molecular, Complete Ionic, and Net Ionic Equations Science, Chemistry Magnesium Chloride Ionic Equation Mgcl 2 can be extracted from. The balanced equation will be calculated along. To find the formula of an ionic compound, first. Web enter an equation of an ionic chemical equation and press the balance button. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. Web electrolysis of magnesium chloride. A magnesium. Magnesium Chloride Ionic Equation.
From colouremployer8.gitlab.io
Marvelous Writing Net Ionic Equations Calculator Photosynthesis And Magnesium Chloride Ionic Equation To find the formula of an ionic compound, first. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web electrolysis of magnesium chloride. Web enter an equation of an ionic chemical equation and press the balance button. Web. Magnesium Chloride Ionic Equation.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Magnesium Chloride Ionic Equation Mgcl 2 can be extracted from. Web electrolysis of magnesium chloride. To find the formula of an ionic compound, first. Web enter an equation of an ionic chemical equation and press the balance button. A magnesium ion has a 2+ charge, while a chlorine ion has a. Web the magnesium chloride formula is derived when magnesium and chloride ions combine. Magnesium Chloride Ionic Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium Chloride Ionic Equation Web now consider the ionic compound formed by magnesium and chlorine. Web electrolysis of magnesium chloride. Web enter an equation of an ionic chemical equation and press the balance button. Mgcl 2 can be extracted from. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. To find the formula. Magnesium Chloride Ionic Equation.
From studylib.net
Ionic Compounds Naming Magnesium Chloride Ionic Equation Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. Web now consider the ionic compound formed by magnesium and chlorine. Magnesium chloride must be heated until it is molten before it will conduct electricity. To find the formula of an ionic compound, first. Web electrolysis of magnesium chloride. The balanced equation will. Magnesium Chloride Ionic Equation.
From tabithadesnhpotts.blogspot.com
Magnesium Chloride and Sodium Hydroxide Net Ionic Equation Magnesium Chloride Ionic Equation Web electrolysis of magnesium chloride. To find the formula of an ionic compound, first. Web a complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Magnesium chloride must be heated until it is molten before it will conduct electricity. Web enter an equation of an ionic chemical equation and press the. Magnesium Chloride Ionic Equation.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Chloride Ionic Equation Magnesium chloride must be heated until it is molten before it will conduct electricity. To find the formula of an ionic compound, first. The balanced equation will be calculated along. Web now consider the ionic compound formed by magnesium and chlorine. Web the magnesium chloride formula is derived when magnesium and chloride ions combine to form the ionic. A magnesium. Magnesium Chloride Ionic Equation.